Coronary blood vessel development
THe endothelial cells that line coronary blood vessels arise primarily from two progenitor sources: the sinus venosus and endocardium (Red-Horse et al., 2010). Dual progenitors are a feature of several developmental systems (Das and Red-Horse, 2017), and Our lab uses the coronaries to study how and why.
Distinct molecular pathways guide progenitor angiogenesis
Sinus venosus-derived coronary angiogenesis, but not that from the endocardium, relies on the growth factor Vegf-C and the peptide hormone Elabela (Chen*, Sharma*, 2014b; Sharma, 2017). Vegfc, Ccbe1, and Elabela are expressed by the epicardium on the surface of the heart where sinus venosus sprouts emerge, and sinus venosus-derived endothelial cells express high levels of the Elabela receptor, Apj or Aplnr. Interestingly, evidence suggests sinus venosus angiogenesis is genetically timed while endocardial sprouting is stimulated by environmental conditions. The endocardial angiogenesis activator (Vegfa) is hypoxia-inducible, while the SV activators are not, and only endocardial sprouts first emerge into hypoxic tissues (Sharma, 2017).
Biological advantages of two progenitor sources
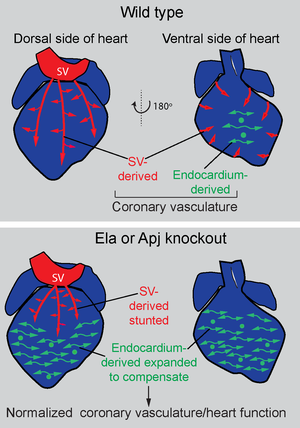
Compensation: One hypothesis for why coronaries have multiple origins is that it could provide an alternative progenitor pool if one source is disrupted. Performing endocardial lineage tracing in Apj/Aplnr mutants revealed that endocardial-derived vessels expand when sinus venosus angiogenesis is deficient (Sharma, 2017).
Support of rapid tissue growth and ventricular compaction: Another hypothesis for the importance of multiple coronary progenitors is that it allows for faster vascularization that could better support rapid growth of the heart wall. Coincident with the onset of coronary angiogenesis, the ventricular heart wall begins transforming from loosely packed trabeculae to thick, compact muscle. This transformation is called ventricular compaction, and, when defective in humans, causes a cardiomyopathy termed Left Ventricular Non-Compaction. Pan endothelial-specific deletion of the chromatin remodeler, Ino80, causes ventricular non-compaction in mice due to defective coronary angiogenesis, while progenitor-specific deletion results in slower heart wall growth and milder non-compaction (Rhee*, Chung*, 2018). Coronary endothelial cells support heart wall growth by secreting paracrine factors such as Col15a1, but, under diseased conditions, the cells produce factors that inhibit cardiomyocyte proliferation (Rhee*, Paik*, 2021). This work indicates that angiogenesis from two coronary progenitor pools promotes optimal expansion of the compact heart wall.
Sinus venosus- and endocardium-derived coronaries ultimately converge
Do cells from different progenitors “remember” their origins and, if so, does this influence cell behavior. We began addressing this question by assessing cell states in single-cell RNA sequencing data from hearts containing sinus venosus or endocardium lineage labels. We found that, early in development, coronary endothelial cells did retain transcriptional signatures of their progenitor, but that this is lost later in development. Instead, transcriptional heterogeneity at later embryonic stages is dictated by spatial localization in the heart. In the adult, cells from both sources have completely converged, and respond similarly to cardiac injury. These results underscore the plasticity of endothelial cells during development, and suggest that early stage samples could identify coronary progenitors in humans (Phansalkar, 2021).
Ischemic heart disease is the number one cause of death worldwide, impacting a growing number of human populations at a wide range of economies (Libby, 2021). It is most commonly cause by disease of the coronary arteries. We hypothesize that studying how developmental systems establish and grow coronary arteries will reveal ways of regenerating them in diseased hearts. Thus, much of our focus is specifically on coronary artery developmental mechanisms.
Discoveries enabled by single-cell analysis
Pre-artery cells build coronary arteries: Using single-cell RNA sequencing, we discovered that a population of single cells within the immature vessel plexus are pre-specified during mid-gestation and subsequently go on to build a major portion of coronary arteries (Su*, Stanley*, Sinha*, 2018). Analyzing the vein to artery cell fate switch at single cell resolution revealed two novel and unexpected features: (1) The vein to artery conversion is gradual and overlapping until a transcriptional threshold is reached forming pre-specified artery cells, and (2) This arterial pre-specification threshold is actively inhibited by cell cycle activation induced by the vein specifying transcription factor, COUP-TF2. Understanding artery development at this new level of detail has implications for strategizing coronary artery regeneration in the diseased or injured heart.
Human and mouse coronary development are highly similar: Reference mapping between mouse and human single-RNA sequencing datasets revealed the conservation of multiple aspects between the two species, including the capillary—pre-artery—artery differentiation trajectory and the location specific heterogeneity where septal vessels experience delayed full perfusion (Phansalkar, 2021). Select differences, such as neurotransmitter receptor expression in humans, could underly structural differences between the two species. Overall, the data validates mouse as a good model system for human coronary development.
Relationship of artery development to blood flow
Since artery endothelial cells differentiation is coupled to cell cycle exit (Su*, Stanley*, Sinha*, 2018), arterial blood vessels must recruit cells inward in order to grow in size. The vasculature has solved this problem by coupling artery differentiation to the propensity to migrate against the direction of blood flow. In this scenario, pre-artery cells differentiate outside of arterial vessels, usually in the vascular plexus or capillary beds, and then migrate upstream into growing arteries. This retrograde flow of endothelial cells during vascular development has been observed in multiple vessel beds and organisms, but the mechanisms directing this behavior are only beginning to be revealed (Red-Horse and Siekmann, 2019).
We found two transcription factors that are involved in endothelial responses to blood flow—Dach1 and SMADs. Dach1 enhances artery endothelial cell differentiation and stimulates their polarization, alignment, and migration against the direction of blood flow, both in culture and the mouse vasculature (Chang, 2017; Raftrey, 2021). Accordingly, Dach1-deficient mice have small coronary arteries, and Dach1-overexpression elongates artery branches. This activity is mediated in part through induction of the chemokine Cxcl12. SMAD activity is required for endothelial cells to align and migrate against the direction of flow, but leaves the polarization response intact (Poduri, 2017) .
Injury Responses and Regeneration
Developing organisms create tissues de novo, and the underlying instructions could inform organ regeneration. In two studies, we have demonstrated that learning basic developmental concepts can inform new regeneration strategies in the heart.
Neonatal heart regeneration and collateral arteries:
We found the developing neonatal mouse heart to have a unique ability to quickly and robustly grow collateral arteries in response to experimental myocardial infarction (Das*,Goldstone*, 2019). Collateral arteries are arteries that bridge two conventional arterial branches and provide blood flow to occluded vessels (i.e. natural bypasses). Myocardial infarction in neonates caused tissue hypoxia, which stimulated the expression of the migratory cue CXCL12 by capillaries situated between injured and healthy arteries. These capillaries become a reparative niche by attracting artery cells that express the CXCL12 receptor, CXCR4, and supporting their transformation into collateral arteries. Remarkably, the neonatal heart can fully regenerate, which is lost in adulthood, and our work showed that its capacity to form collateral arteries is one reason why. We injected CXCL12 into injured adult hearts and found that this re-activated collateral development, presenting the exciting possibility that knowledge of developmental processes could identify reparative pathways.
Effects of Dach1 overexpression during injury:
Because Dach1 was a transcription factor required for coronary artery growth during development that increased arterial branches when overexpressed (see above), we investigates the effect of inducing it in adult mice during cardiac injury. This manipulation increased animal survival and heart function after myocardial infarction (Raftrey, 2021). Candidates for this protective activity are increased arteries and vascular connections post-injury or Dach1’s effect on lipid metabolism pathways.
Imaging and functional assessment of blood flow (in collaboration with Dr. Alison Marsden)
The most important parameter of collateral arteries is their capacity to restore blood flow, which we most commonly assessed by observing the distribution of injected fluorescent dyes. Due to advances in tissue clearing and light sheet imaging, we are now able to us computational flow modeling (CFD) to: 1. Obtain high resolution estimates of blood flow and 2. Create models where multiple collateral configurations can be tested. Our data predict that naturally forming collaterals in neonatal hearts perform very well at restoring blood flow. Naturally occurring collaterals in adults hearts perform very poorly while or those induced by CXCL12 are somewhere in between. This is because of the morphology of the coronary artery tree and how is expands during post-natal growth.